GCP
Our experienced team of auditors can cover auditing services across the various phases of development and complexities of a clinical study. Our GCP QA auditing services can help you to ensure that your clinical trials are compliant with all applicable GCP regulations. The auditors also focus on integrity of the process involved. In a rapidly changing world of clinical research, GCP QA audits must be stringent and are becoming progressively challenging. In that respect, RiverArk has dedicated auditors to tackle challenges in every aspect, including either physically attending investigator sites, or conducting the required audit remotely. In short, a measured approach towards the situation at hand. We offer a wide spectrum of services in Good Clinical Practices according to the ICH-GCP E6 (R2) guideline.
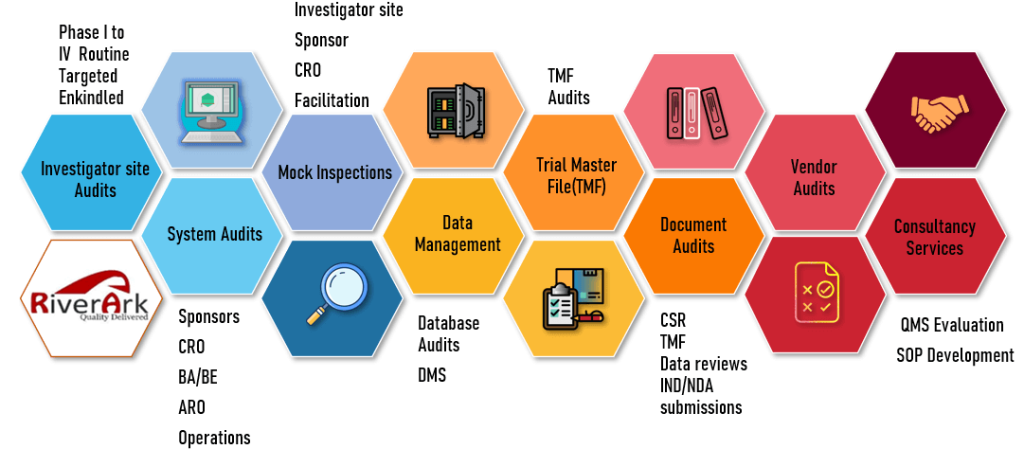
GCP Audits
- Investigator Site Audits – Phase 1 to 4, Routine, Targeted, For cause
- Systems Audits – Sponsors, CRO, BA/BE, ARO, Operations
- Mock Inspections Audits– Investigator site, Sponsor, CRO, Facilitation
- Data Management Audits – Database Audits, DMS
- Trial Master File (TMF) Audits
- Document Audits – CSR, Data reviews, IND/NDAVendor Audits
- Vendor Audits